V-Ensure Pharma Hiring for Regulatory Affairs, QA, QC, Micro, Warehouse, Production, AR&D, FR&D on 2nd June 2024[Freshers]
About Company
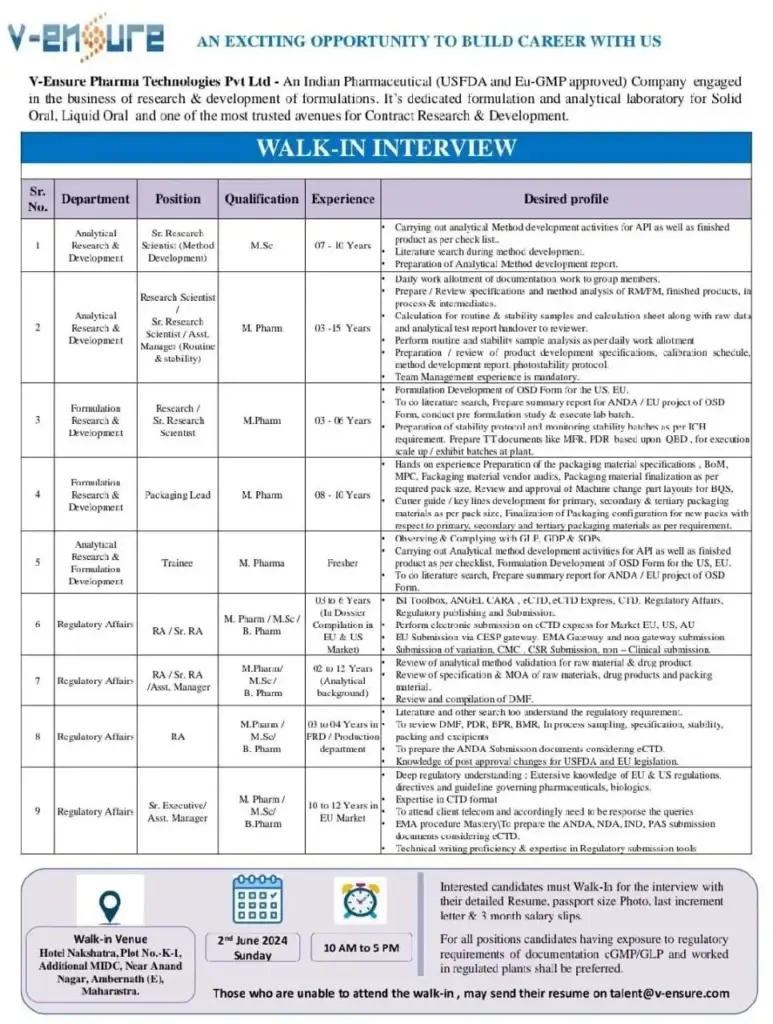
Walk-in interview with V-Ensure Pharma Technologies Pvt. Ltd. in 2024.Pharma Technologies Pvt. Ltd., V-Ensure complete notification information is below.Candidates that meet the requirements can show up for the interview at the designated time and location.a reputable pharmaceutical firm that develops, produces, and distributes oral solid dosage forms in a regulated market. For our USFDA and EU-GMP authorized OSD manufacturing facility located at N-32, Additional Patalganga MIDC, Tal.-Panvel, Dist.-Raigad, Maharashtra, we are seeking capable, growth-oriented applicants.
Position Name :QA, QC, Micro, Warehouse, Production, AR&D, FR&D, Regulatory Affairs
Organization :V-Ensure Pharma
Qualification:Any Graduate /Diploma/ ITI/B. Pharm / M.Pharm /M.Sc
Experience:0 to 12 years
Salary:N/A
Location:Maharastra
Job Vacancy Details
Company: V-Ensure Pharma Technologies Pvt. Ltd.
Location: Maharashtra
Department: QA, QC, Micro, Warehouse, Production, AR&D, FR&D, Regulatory Affairs
Positions Available:
- Operator
- Sr. Operator
- Officer
- Sr. Officer
- Executive
- RA
- Sr. RA
- RS
- Sr. RS
Qualifications:
- Any Graduate
- Diploma
- ITI
- B. Pharm
- M. Pharm
- M.Sc
Experience: 0 to 12 years
Job Description:
Join a reputed pharmaceutical company involved in the development, manufacturing, and marketing of oral solid dosage forms for regulated markets. We seek competent, growth-oriented candidates for our USFDA & EU-GMP approved OSD manufacturing facility at N-32, Additional Patalganga MIDC, Tal.- Panvel, Dist.- Raigad.
Selection Process: Interview
Mode of Interview: Face-to-face
Interview Rounds: HR
Walk-In Drive Details:
- Date: 2nd June 2024
- Time: 10 AM to 5 PM
- Venue: Hotel Nakshatra, Plot No K-1, Additional MIDC, Near Anandnagar, Ambernath, Maharashtra
Important Notes:
- Interested candidates must bring their Resume, Passport Size Photo, Last Increment Letter, and 3 Months Salary Slips.
- Preference will be given to candidates with experience in regulatory requirements of documentation cGMP/GLP and those who have worked in regulated plants.
- Those unable to attend the walk-in may send their updated resume to [email protected]. Please mention the applied section name in the subject line.https://chat.whatsapp.com/DO9PMPj2H3K4SikyHri52x
To get More Job Updates through Telegram Join in below link
https://t.me/careerpathwayjobs
To get Today all post click below link
CareerPathway – Guide to your sucess