FINOSO Pharma Hiring 2025: Leadership Opportunities in Global CDMO Expansion
FINOSO Pharma, a pioneering and rapidly growing Global Contract Development and Manufacturing Organization (CDMO), has announced an exciting opportunity for seasoned pharmaceutical professionals to join its leadership team. As FINOSO continues its strategic global expansion, it is focused on strengthening its Formulation R&D and Analytical R&D divisions by hiring visionary leaders with a proven record of driving innovation and excellence.
Why Join FINOSO Pharma?
FINOSO stands at the forefront of pharmaceutical development with its unwavering commitment to precision, performance, and partnership. With a solid foundation in developing oral solid dosage forms, ophthalmics, and nasal sprays, the company serves regulated markets across the EU, US, and Canada. The organization offers a platform where leaders can create a global impact, backed by cutting-edge infrastructure, a talented workforce, and world-class research capabilities.
Open Leadership Positions at FINOSO Pharma
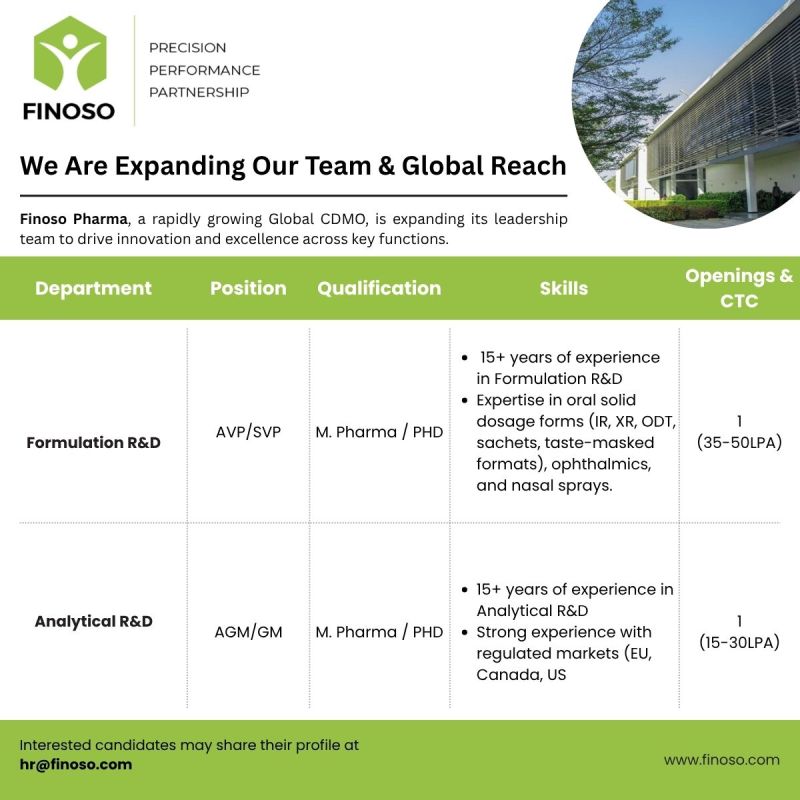
1. AVP/SVP – Formulation R&D
Department: Formulation Research & Development
Position Level: Associate Vice President / Senior Vice President
Location: Not disclosed (expected to be at a strategic R&D facility)
CTC Range: ₹35–50 LPA
Openings: 1
Qualifications Required
- M. Pharma or Ph.D. in Pharmaceutics or related discipline
Key Skills & Responsibilities
- 15+ years of experience in Formulation R&D, particularly in oral solid dosage systems.
- Deep expertise in:
- Immediate Release (IR)
- Extended Release (XR)
- Orally Disintegrating Tablets (ODT)
- Taste-masked formats
- Sachets
- Proven exposure in ophthalmic and nasal spray formulations.
- Leadership in technology transfer, scale-up, and regulatory documentation.
- Ability to manage cross-functional teams and collaborate with marketing, QA/QC, and regulatory affairs.
2. AGM/GM – Analytical R&D
Department: Analytical Research & Development
Position Level: Assistant General Manager / General Manager
Location: Not disclosed (likely at central analytical labs)
CTC Range: ₹15–30 LPA
Openings: 1
Qualifications Required
- M. Pharma or Ph.D. in Pharmaceutical Analysis or related fields
Key Skills & Responsibilities
- 15+ years of hands-on experience in Analytical Method Development and Validation
- Strong familiarity with regulatory requirements and compliance for EU, Canada, and US markets
- Proficiency in techniques such as:
- HPLC
- GC
- Dissolution testing
- Stability studies
- Experience with:
- Data integrity and 21 CFR Part 11 compliance
- ICH guidelines
- Raw data documentation and audit preparedness
Ideal Candidate Profile
To thrive in FINOSO’s high-performance environment, candidates must demonstrate:
- Proven success in regulatory submissions (ANDA, NDA, DMF)
- Collaborative and strategic thinking
- Passion for continuous research innovation
- Strong communication skills for cross-border coordination
- Deep commitment to quality assurance and regulatory compliance
What Makes FINOSO an Employer of Choice?
Global Reach with Local Excellence
With a global outlook and state-of-the-art facilities, FINOSO enables its R&D teams to develop formulations and analytical strategies tailored for international markets, ensuring maximum regulatory and market acceptance.
Empowering Work Culture
FINOSO fosters a workplace culture that values knowledge sharing, employee development, and innovation-centric leadership. Every individual is empowered to contribute significantly to product development pipelines that address unmet clinical needs.
Attractive Compensation and Growth
- Highly competitive salary packages (up to ₹50 LPA for top-tier roles)
- Performance bonuses and leadership incentives
- Exposure to global project teams and international collaborations
How to Apply
If you are ready to take on a leadership role that influences pharmaceutical development on a global scale, send your profile to:
📧 [email protected]
🌐 Visit www.finoso.com for more details.
Make sure to include:
- Updated CV with detailed experience
- Cover letter highlighting your leadership in R&D
- List of successful regulatory submissions (if applicable)
- Notice period and current CTC
Conclusion: Take the Leap Towards Global Pharmaceutical Leadership
With its firm commitment to scientific excellence, regulatory compliance, and patient-centric innovation, FINOSO Pharma provides the ideal platform for seasoned pharma professionals to scale new heights in their careers. Whether you specialize in formulation design or analytical development, this is your chance to lead innovation from the front lines of one of the most dynamic Global CDMOs.
Step into a future where your expertise shapes healthcare worldwide. Don’t miss this opportunity to be part of a company that’s redefining pharmaceutical development for the next generation of therapeutics.
To Join In Whatsapp group Click below Link
https://chat.whatsapp.com/ERzsuXoDDnOLBZ3spkddn5
To Join Telegram Channel Click Below Link
https://t.me/careerpathwayjobs
To know the Latest Job updates click below Link
Career Pathway – Guide to your success
