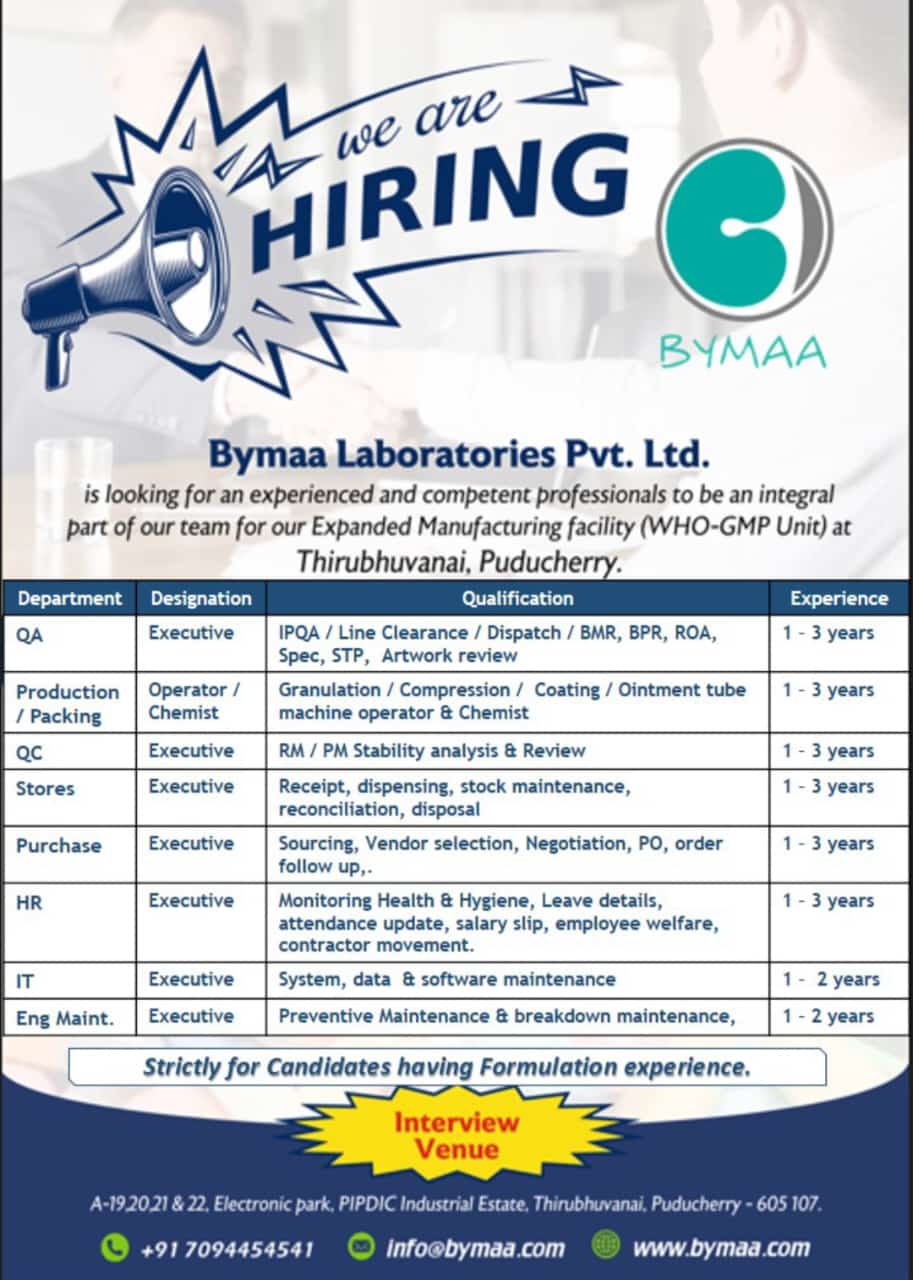
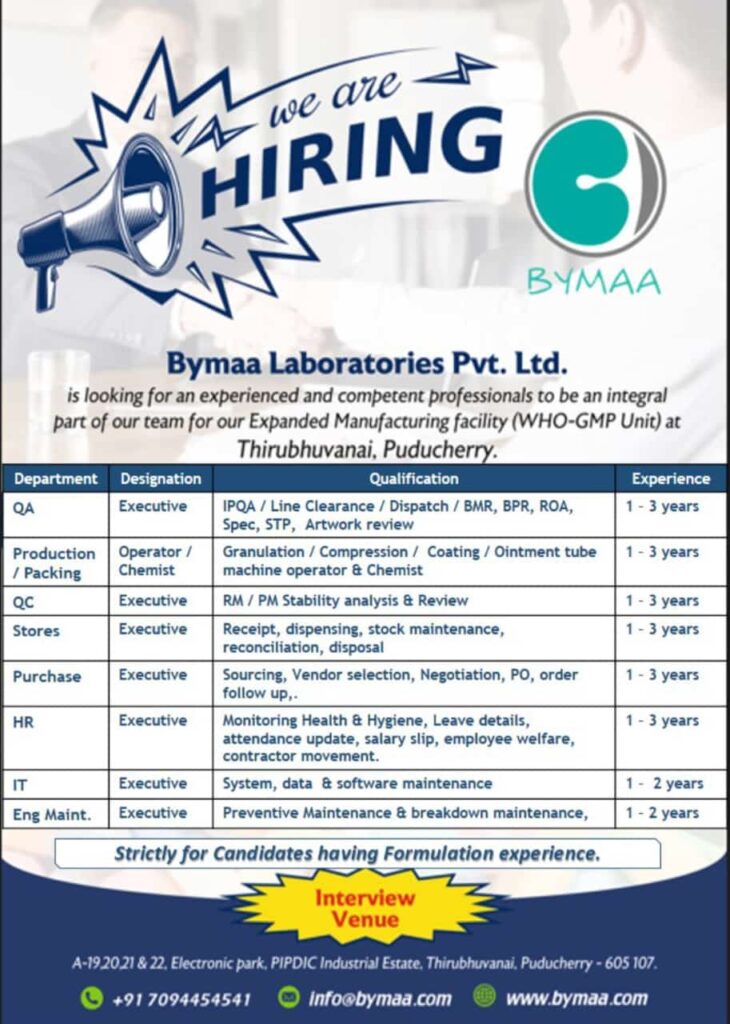
Bymaa Laboratories Pvt. Ltd Hiring for Multiple Positions at Puducherry
Bymaa Laboratories Pvt. Ltd., a trusted name in pharmaceutical manufacturing, is seeking skilled professionals to join its expanding team at the WHO-GMP-compliant manufacturing facility in Thirubhuvanai, Puducherry. With various roles across departments such as Quality Assurance, Production, and IT, Bymaa Laboratories offers opportunities for those with pharmaceutical formulation experience looking to advance their careers.
About Bymaa Laboratories Pvt. Ltd.
Bymaa Laboratories Pvt. Ltd. specializes in high-quality pharmaceutical products, adhering to stringent industry standards. With a focus on innovation and quality, Bymaa Laboratories is a fast-growing company that prides itself on its state-of-the-art WHO-GMP certified manufacturing facility and a professional work environment conducive to growth and learning.
Overview of Open Positions
The following positions are available for individuals with experience in pharmaceutical formulation. Bymaa Laboratories seeks dedicated professionals in Quality Assurance, Production, Quality Control, and more.
Quality Assurance (QA) Executive
Responsibilities:
- Oversee In-Process Quality Assurance (IPQA), Line Clearance, Dispatch activities.
- Review of Batch Manufacturing Records (BMR), Batch Packing Records (BPR), Records of Analysis (ROA), Specifications (Spec), and Standard Test Procedures (STP).
- Conduct artwork review.
Experience Required: 1-3 years in quality assurance within a formulation environment.
Production/Packing Operator or Chemist
Responsibilities:
- Operate machinery for Granulation, Compression, and Coating, including ointment tube machines.
- Maintain precise control over production and packing processes.
Experience Required: 1-3 years of experience as an operator or chemist in production.
Quality Control (QC) Executive
Responsibilities:
- Execute Raw Material (RM) and Packaging Material (PM) stability analysis.
- Review and ensure quality compliance of materials used in production.
Experience Required: 1-3 years in Quality Control, with proficiency in stability analysis.
Stores Executive
Responsibilities:
- Oversee receipt, dispensing, and stock maintenance.
- Reconcile inventories and manage disposal processes.
Experience Required: 1-3 years in inventory and stock management.
Purchase Executive
Responsibilities:
- Handle sourcing, vendor selection, and negotiation.
- Oversee purchase orders (PO) and order follow-up.
Experience Required: 1-3 years in procurement, with a strong focus on vendor relations.
HR Executive
Responsibilities:
- Monitor health and hygiene standards.
- Update employee attendance and leave records.
- Handle salary slips, welfare activities, and contractor movements.
Experience Required: 1-3 years in HR management, preferably in a manufacturing setting.
IT Executive
Responsibilities:
- Maintain and support systems, data, and software used across departments.
Experience Required: 1-2 years in IT maintenance within a regulated environment.
Engineering Maintenance (Eng Maint.) Executive
Responsibilities:
- Conduct preventive and breakdown maintenance on critical equipment.
Experience Required: 1-2 years in equipment maintenance, focusing on preventive care and troubleshooting.
Why Choose a Career with Bymaa Laboratories?
Bymaa Laboratories offers a structured environment that values precision, quality, and safety. Working here provides you with exposure to advanced pharmaceutical practices, ongoing skill development, and an opportunity to contribute to quality healthcare.
Job Requirements and Essential Skills
Each role demands specific technical knowledge, problem-solving abilities, and teamwork skills. All candidates are expected to have pharmaceutical formulation experience, ensuring they bring a foundational understanding of industry practices.
Interview Details and Venue
Candidates interested in joining Bymaa Laboratories can attend a walk-in interview at the following venue:
- Address: A-19, 20, 21 & 22, Electronic Park, PIPDIC Industrial Estate, Thirubhuvanai, Puducherry – 605107.
- Contact: +91 7094454541
- Email: info@bymaa.com
- Website: www.bymaa.com
Preparation Tips for Job Applicants
- Familiarize with Bymaa Laboratories: Understand the company’s mission, values, and products to align your responses during the interview.
- Review WHO-GMP Guidelines: Knowledge of WHO-GMP standards is essential, as Bymaa’s facility complies with these regulations.
- Demonstrate Teamwork and Technical Skills: Each role requires collaboration, so be ready to discuss experiences where you worked effectively with others.
Understanding WHO-GMP Standards
WHO-GMP (World Health Organization – Good Manufacturing Practice) standards are a benchmark in pharmaceutical quality. Compliance ensures that every product is safe, effective, and consistently produced. Bymaa’s commitment to WHO-GMP underscores its focus on high-quality, reliable products.
Life in Puducherry’s Pharmaceutical Industry
Puducherry is a growing hub for the pharmaceutical sector, with many companies establishing operations due to the state’s supportive policies and infrastructure. A career here allows professionals to engage in a dynamic, fast-evolving industry, offering stability and opportunities for progression.
Conclusion
Bymaa Laboratories Pvt. Ltd. presents a unique opportunity for professionals with pharmaceutical formulation experience. Joining their WHO-GMP-compliant facility in Puducherry means becoming part of a team that values quality, innovation, and career development. If you meet the qualifications, this walk-in interview could be your pathway to a rewarding career in pharmaceutical manufacturing.
To Join In Whatsapp group Click below Link
https://chat.whatsapp.com/ERzsuXoDDnOLBZ3spkddn5
To Join Telegram Channel Click Below Link
https://t.me/careerpathwayjobs
To know the Latest Job updates click below Link
Career Pathway – Guide to your success