Brooks Steriscience Walk-In Interview 2025 – Hiring for Multiple API Manufacturing & QA Roles
Are you a pharmaceutical professional looking for the next big career opportunity? Brooks Steriscience Limited, a leading name in the Active Pharmaceutical Ingredients (API) manufacturing sector, is conducting a walk-in interview on 1st June 2025 in Vadodara, Gujarat. If you have hands-on experience in API manufacturing, documentation, warehousing, QMS, or validation, this is your golden chance!
🏢 About Brooks Steriscience Limited
Located in Karjan Taluka, Vadodara, Brooks Steriscience Limited is renowned for its advanced pharmaceutical manufacturing infrastructure and commitment to quality, regulatory compliance, and innovation.
🗓️ Walk-In Interview Details
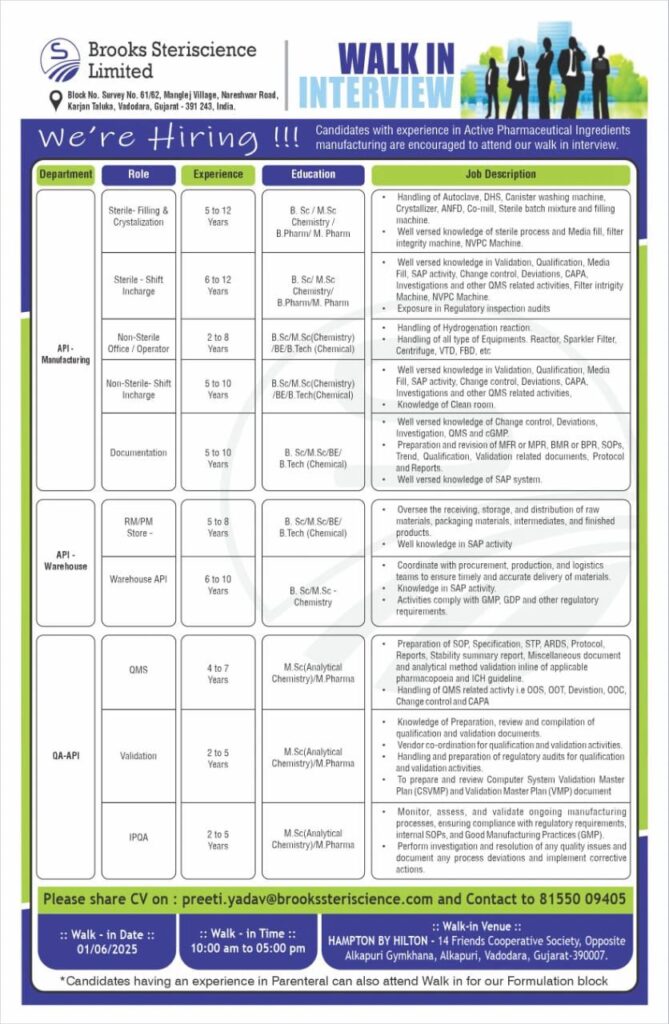
Date: 1st June 2025
Time: 10:00 AM – 5:00 PM
Venue:
HAMPTON BY HILTON,
14 Friends Cooperative Society, Opposite Alkapuri Gymkhana, Alkapuri, Vadodara, Gujarat – 390007
🧑💼 Openings by Department
🔬 API – Manufacturing
| Role | Experience | Education | Key Responsibilities |
|---|---|---|---|
| Sterile – Filling & Crystallization | 5 to 12 Years | B.Sc / M.Sc Chemistry / B.Pharm / M.Pharm | Handling of sterile processes, ANFD, DHS, autoclave, crystallizer; strong knowledge of cleanroom and MVPC Machine |
| Sterile – Shift Incharge | 6 to 12 Years | B.Sc / M.Sc Chemistry / B.Pharm / M.Pharm | Handling validations, media fill, change controls, CAPA, investigations; exposure in regulatory inspections |
| Non-Sterile – Office / Operator | 2 to 8 Years | B.Sc/M.Sc (Chemistry) / BE/B.Tech (Chemical) | Handling hydrogenation, sparkler filter, VTD, FBD, etc. |
| Non-Sterile – Shift Incharge | 5 to 10 Years | B.Sc/M.Sc (Chemistry) / BE/B.Tech (Chemical) | Responsible for validations, deviations, CAPA, documentation |
| Documentation | 5 to 10 Years | B.Sc/M.Sc/BE/B.Tech (Chemical) | Change controls, SOP preparation, deviation reporting, regulatory audits |
🏢 API – Warehouse
| Role | Experience | Education | Responsibilities |
|---|---|---|---|
| RM/PM Store | 5 to 8 Years | B.Sc/M.Sc/BE/B.Tech (Chemical) | Receiving & storing raw materials, SAP, GDP compliance |
| Warehouse API | 6 to 10 Years | B.Sc/M.Sc Chemistry | Inventory management, logistics coordination, SAP usage |
📑 QA – API (Quality Assurance)
| Role | Experience | Education | Responsibilities |
|---|---|---|---|
| QMS | 4 to 7 Years | M.Sc (Analytical Chemistry) / M.Pharm | SOPs, change controls, CAPA, audit support |
| Validation | 2 to 5 Years | M.Sc (Analytical Chemistry) / M.Pharm | Validation protocols, CSVP, VMP, audit readiness |
| IPQA | 2 to 5 Years | M.Sc (Analytical Chemistry) / M.Pharm | In-process checks, GMP compliance, deviation management |
📌 Important Instructions
📧 Email Your CV: [email protected]
📞 Contact: 81550 09405
🧪 Candidates with Parenteral experience are highly encouraged
📂 Bring updated resume, certificates, and relevant documents
🚀 Why Join Brooks Steriscience?
Cutting-edge API manufacturing environment
Strong focus on global quality and regulatory standards
Growth-oriented culture with challenging roles
Opportunities in both Sterile and Non-Sterile segments
Don’t miss this chance to grow your pharmaceutical career with one of the most promising API manufacturers in India. Be there at the Brooks Steriscience Walk-In Drive on 1st June 2025 and unlock your path to success!
🔗 For more updates, keep visiting: www.brookssteriscience.com
#PharmaJobs #WalkInInterview #APIManufacturing #VadodaraJobs #BrooksSteriscience
To Join In Whatsapp group Click below Link
https://chat.whatsapp.com/ERzsuXoDDnOLBZ3spkddn5
To Join Telegram Channel Click Below Link
https://t.me/careerpathwayjobs
To know the Latest Job updates click below Link
Career Pathway – Guide to your success


