Brooks Steriscience Hiring: Multiple Roles in API Manufacturing, QA, QC & More | Vadodara
Brooks Steriscience is expanding its team and is looking for talented professionals to join our facility in Vadodara, Gujarat. We have multiple openings across various departments, including API Manufacturing, QA-IPQA, QA-QMS, QC, and IT. If you have experience in the pharmaceutical industry and are looking for a challenging and rewarding career, we encourage you to apply.
Exciting Career Opportunities at Brooks Steriscience, Vadodara
Join Brooks Steriscience, a leading pharmaceutical company committed to excellence and quality. We offer a dynamic work environment and opportunities for professional growth. Explore our open positions and take the next step in your career.
Open Positions Across Various Departments
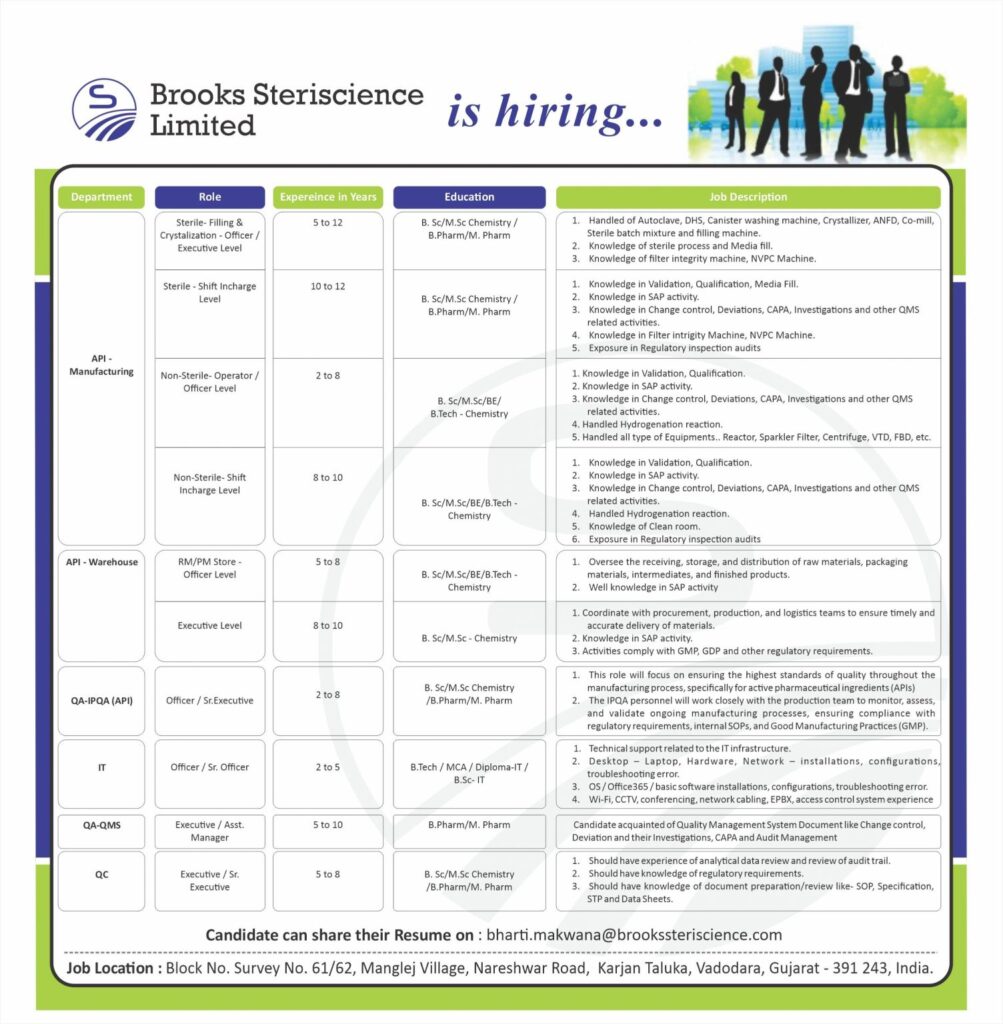
API Manufacturing Roles
Sterile-Filling & Crystalization-Officer/Executive Level (5-12 Years)
Experience in handling Autoclave, DHS, Canister washing machine, Crystallizer, ANFD, Co-mill, Sterile batch mixture, and filling machine. Knowledge of sterile processes and Media fill. Filter integrity machine and NVPC Machine.
Sterile Shift Incharge Level (10-12 Years)
Knowledge in Validation, Qualification, Media Fill, SAP activity, Change control, Deviations, CAPA, Investigations, Filter integrity machine, NVPC Machine, and Regulatory inspection audits.
Non-Sterile-Operator/Officer Level (2-8 Years)
Knowledge in Validation, Qualification, SAP activity, Change control, Deviations, CAPA, Investigations, Hydrogenation reaction, and handling various equipment like Reactor, Sparkler Filter, Centrifuge, VTD, FBD, etc.
Non-Sterile-Shift Incharge Level (8-10 Years)
Knowledge in Validation, Qualification, SAP activity, Change control, Deviations, CAPA, Investigations, Hydrogenation reaction, clean room, and Regulatory inspection audits.
API-Warehouse Roles
RM/PM Store-Officer Level (5-8 Years)
Overseeing the receiving, storage, and distribution of raw materials, packaging materials, intermediates, and finished products. Knowledge in SAP activity.
Executive Level (8-10 Years)
Coordinating with procurement, production, and logistics teams, knowledge in SAP activity, and ensuring compliance with GMP, GDP, and other regulatory requirements.
QA-IPQA (API) Roles
Officer/Sr. Executive (2-8 Years)
Ensuring quality throughout the manufacturing process for APIs, monitoring, assessing, and validating manufacturing processes, and ensuring compliance with regulatory requirements and GMP.
IT Roles
Officer/Sr. Officer (2-5 Years)
Providing technical support for IT infrastructure, hardware, network installations, configurations, troubleshooting, software installations, and managing WI-FI, CCTV, conferencing, network cabling, EPBX, and access control systems.
QA-QMS Roles
Executive/Asst. Manager (5-10 Years)
Knowledge of Quality Management System documents like Change control, Deviation, Investigations, CAPA, and Audit Management.
QC Roles
Executive/Sr. Executive (5-8 Years)
Experience in analytical data review, knowledge of regulatory requirements, and document preparation/review like SOP, Specification, STP, and Data Sheets.
How to Apply
Candidates can share their resumes at: [email protected]
Job Location
Block No. Survey No. 61/62, Manglej Village, Nareshwar Road, Karjan Taluka, Vadodara, Gujarat – 391 243, India.
To Join In Whatsapp group Click below Link
https://chat.whatsapp.com/ERzsuXoDDnOLBZ3spkddn5
To Join Telegram Channel Click Below Link
https://t.me/careerpathwayjobs
To know the Latest Job updates click below Link
Career Pathway – Guide to your success


