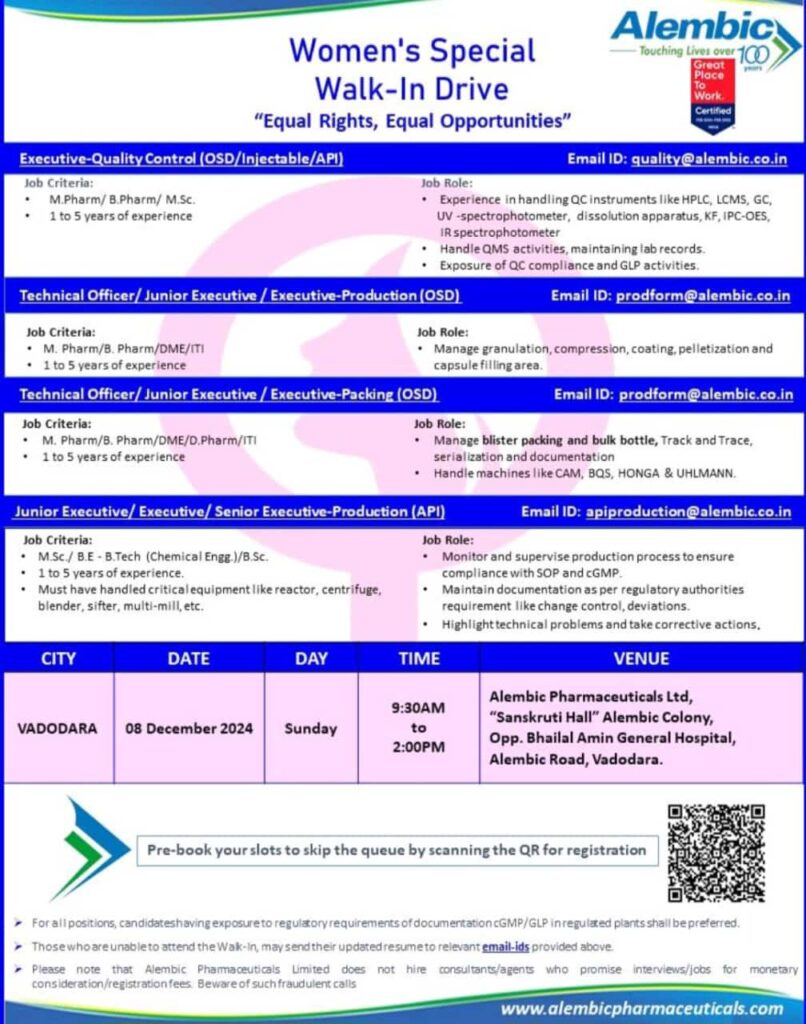
Alembic Pharmaceuticals Women’s Special Walk-In Drive
Alembic Pharmaceuticals is organizing a Women’s Special Walk-In Drive to empower talented women professionals. This initiative offers diverse roles across Quality Control, Production, and Packing departments. If you have the passion and skills, don’t miss this chance to build your career with a reputed organization.
Walk-In Drive Details
- City: Vadodara
- Date: 8th December 2024, Sunday
- Time: 9:30 AM to 2:00 PM
- Venue:
Alembic Pharmaceuticals Ltd
“Sanskruti Hall,” Alembic Colony, Opp. Bhailal Amin General Hospital, Alembic Road, Vadodara.
Pre-Registration
- Scan the QR code to pre-book your slot and skip the queue.
Available Job Positions and Criteria
Executive – Quality Control (OSD/Injectable/API)
Eligibility:
- Qualification: M.Pharm/B.Pharm/M.Sc.
- Experience: 1 to 5 years.
Key Responsibilities:
- Operate QC instruments like HPLC, LCMS, GC, UV-spectrophotometer, and others.
- Handle Quality Management Systems (QMS) and maintain lab records.
- Ensure compliance with GLP and QC guidelines.
Email for Applications: [email protected]
Technical Officer/Junior Executive/Executive – Production (OSD)
Eligibility:
- Qualification: M.Pharm/B.Pharm/DME/ITI
- Experience: 1 to 5 years.
Key Responsibilities:
- Manage areas like granulation, compression, coating, pelletization, and capsule filling.
Email for Applications: [email protected]
Technical Officer/Junior Executive/Executive – Packing (OSD)
Eligibility:
- Qualification: M.Pharm/B.Pharm/DME/D.Pharm/ITI
- Experience: 1 to 5 years.
Key Responsibilities:
- Handle blister packing, bulk bottle management, serialization, and documentation.
- Operate machines like CAM, BQS, HONGA, and UHLMANN.
Email for Applications: [email protected]
Junior Executive/Executive/Senior Executive – Production (API)
Eligibility:
- Qualification: M.Sc./B.E-B.Tech (Chemical Engineering)/B.Sc.
- Experience: 1 to 5 years.
Key Responsibilities:
- Monitor and supervise the production process while ensuring SOP and cGMP compliance.
- Operate critical equipment such as reactors, centrifuges, blenders, and more.
- Document as per regulatory requirements and resolve technical issues promptly.
Email for Applications: [email protected]
Preferred Skills and Experience
- Exposure to regulatory documentation, cGMP, and GLP standards in regulated environments.
- Hands-on experience in relevant equipment and compliance activities.
Important Notes for Candidates
- If unable to attend the walk-in, you can send your updated resume to the relevant email IDs.
- Alembic Pharmaceuticals does not engage consultants or agents for job interviews or monetary considerations. Beware of fraudulent activities.
Why Join Alembic Pharmaceuticals?
Alembic Pharmaceuticals is a Great Place to Work-certified organization, touching lives across 100+ countries. With cutting-edge facilities and a strong commitment to equality, Alembic offers a rewarding and growth-oriented career path.
Visit: www.alembicpharmaceuticals.com
To Join In Whatsapp group Click below Link
https://chat.whatsapp.com/ERzsuXoDDnOLBZ3spkddn5
To Join Telegram Channel Click Below Link
https://t.me/careerpathwayjobs
To know the Latest Job updates click below Link
Career Pathway – Guide to your success

